APPLICATION
DAIRY INDUSTRY
Industry Hygiene Risks:
Dairy Factory Hygiene Solutions are essential for preventing contamination, ensuring product quality, and maintaining HACCP compliance in modern dairy processing plants. Dairy factories face risks such as biofilm growth, cross-contamination, and manual cleaning errors, making standardized hygiene processes critical.
Manual cleaning is inconsistent and often fails to remove milk fat residues or protein deposits inside pipelines, creating hotspots for biofilms. Equipment with poor hygienic design further increases the difficulty of eliminating microbial harborage points. As regulatory pressure intensifies—driven by HACCP, GMP, and ISO 22000—manufacturers must demonstrate validated sanitation procedures and complete documentation for every cleaning cycle. Additionally, frequent product changeovers require rapid yet fully verified CIP cycles, which many factories fail to monitor effectively.
When cleaning parameters such as time, temperature, chemical concentration, and flow rate are not controlled precisely, contamination risks increase, leading to spoilage, recalls, and regulatory non-compliance. These challenges highlight the urgent need for automated, traceable, and industry-standard cleaning and sanitizing solutions.
Dairy Factory Hygiene Solutions – Key Risks in Dairy Processing
1. Raw Milk Receiving & Storage
High microbial load from raw milk
Contamination from tanks, hoses, and transfer pipes
2. Equipment Internal Surfaces
Protein, lactose, and milk fat buildup
Rapid biofilm formation inside valves, pumps, pipelines
3. Thermal Processing (Pasteurization / HTST / UHT)
Insufficient heating time or temperature
Residues inside heat exchangers
4. Filling & Packaging Area
Product-contact surfaces contaminated during filling
Airborne contamination or operator-related contamination
5. Environmental Contamination
Tools and brushes becoming a contamination source
Moisture transfer between wet and dry zones (critical for powdered dairy)
Why Proper Cleaning & Sanitizing Are Required
Without a proper dairy processing plant cleaning program, biofilms and milk residues quickly accumulate inside tanks, pipelines, and heat exchangers.
CIP (Clean-in-Place) Systems:
Essential for tanks and pipelines that cannot be disassembled. Automated CIP ensures consistent temperature, chemical concentration, and flow rate.Hygienic Design:
Smooth, sloped, crevice-free surfaces prevent soil retention and reduce cleaning complexity.Validation & Verification:
ATP testing, surface swabs, microbial testing, and sanitation logs prove the effectiveness of each cleaning cycle.Regulatory Compliance:
Meeting HACCP, GMP, GMR, and ISO 22000 requirements for sanitation records, CCP controls, and cleaning procedures.
Sanitizing Workflow in Dairy Processing
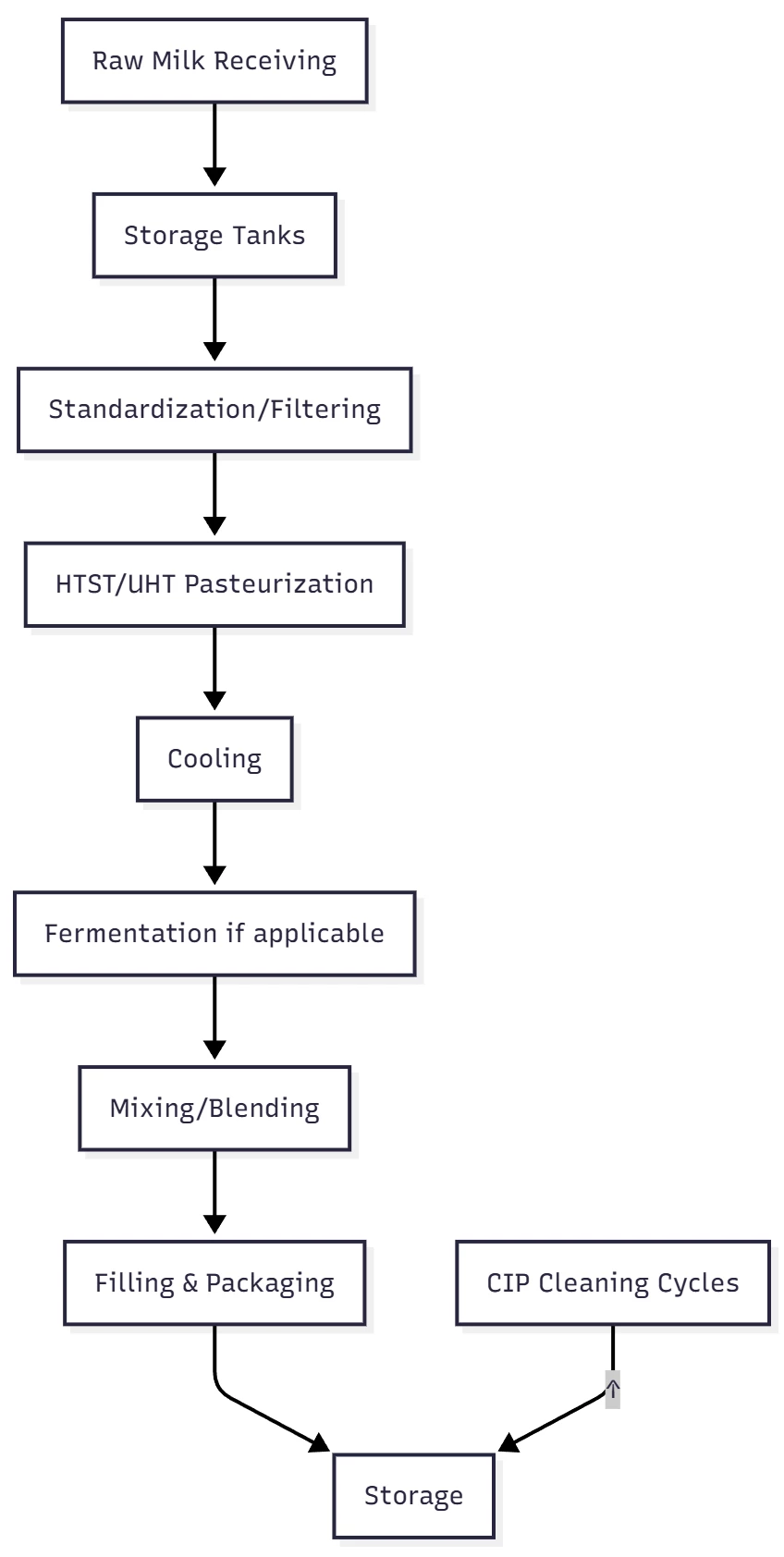
Critical Control Points (CCPs)
These Dairy Factory Hygiene Solutions improve consistency and reduce downtime caused by hygiene failures.
| Process Stage | Hazard | CCP | Critical Limits | Monitoring & Actions |
|---|---|---|---|---|
| Pasteurization/HTST | Pathogens | Temperature & holding time | 72°C for 15s (example) | Continuous monitoring; stop production on deviation |
| CIP Cleaning | Microbial residues | Temp, time, chemical concentration | Based on detergent specs | ATP test, swabbing, chemical verification |
| Filling Zone | Cross-contamination | Hygiene of contact surfaces | Acceptable ATP & microbial levels | Air quality checks, daily sanitizing |
| Tools & Brushes | Biofilm | Cleanliness & storage | Sanitized after each shift | Tool zoning and disinfection program |
Case Study: CIP Optimization in a Yogurt Factory
A large yogurt manufacturer faced recurring microbial failures in the filling area. Investigations showed incomplete CIP cycles and inconsistent enzyme removal in pipelines. After implementing a dual-loop CIP system with automated monitoring of temperature, flow rate, and chemical concentration, the plant introduced ATP verification for each cleaning cycle. Within three months, sanitation failures dropped from 8% to 0.5%, filling-line downtime decreased significantly, and regulatory audit scores improved. The case demonstrates that strong hygienic design, CIP validation, and data-driven sanitation can drastically reduce contamination risks.
Modern dairy processing plant cleaning systems help factories improve audit scores and reduce contamination risks.
Regulations & Standards
HACCP: Hazard analysis, CCP identification, and sanitation monitoring.
GMP / GMR: Cleaning procedures, hygiene plans, equipment calibration, and sanitation logs.
ISO 22000: Structured food safety management system for dairy plants.
Local Regulations: Requirements for raw milk handling, sanitation SOPs, record-keeping, and product traceability.
Essential Hygiene Equipment for Dairy Factory Compliance
Our Dairy Factory Hygiene Solutions cover cleaning-in-place (CIP), hand hygiene, shoe sanitizing, and access control systems.
Hygiene Station Series: /products/hygiene-stations
Boot Washer / Sole Washer: /products/shoe-cleaning
Foam Cleaning System: /products/foam-cleaning





